Scanning probes¶
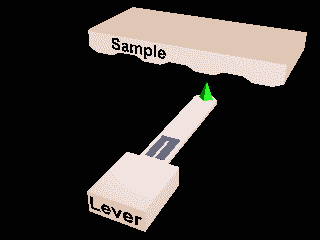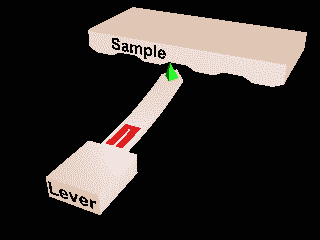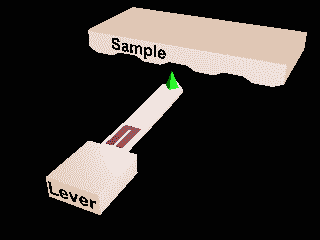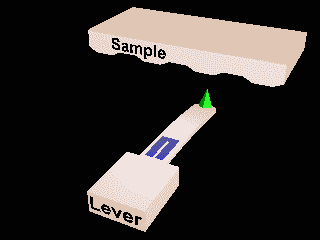 | 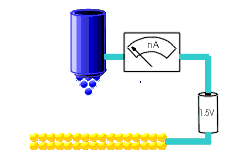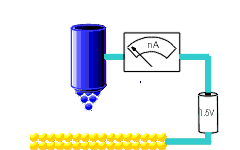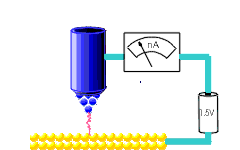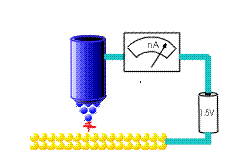 |
Atomic force microscopy and Scanning Tunnelling microscopy.
Local measurements.
Invasive?
Ambient or liquid conditions?
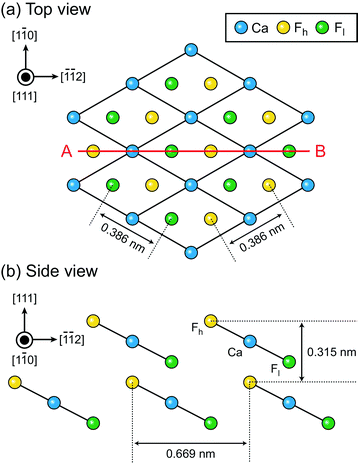
3D Solvation Structures on CaF$_2$¶
UHV measurements at 4K are all very well, but some applications require a more fluid environment
- solar cells
- batteries
- photocathode / anodes
and of course study of material processes like weathering, corrosion, catalysis
3D Solvation Structures on CaF$_2$¶
UHV measurements at 4K are all very well, but some applications require a more fluid environment
- solar cells
- batteries
- photocathode / anodes
and of course study of material processes like weathering, corrosion, catalysis
How do we see water density above a surface?¶
How do we see water density above a surface?¶
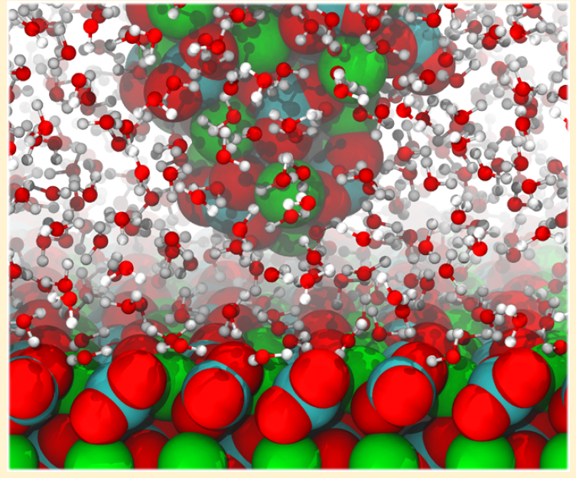
AFM - we'll see that this allows 3D mapping of the interface structure.
2 sided strategy: manipulate both experiment and simulation data for comparison¶
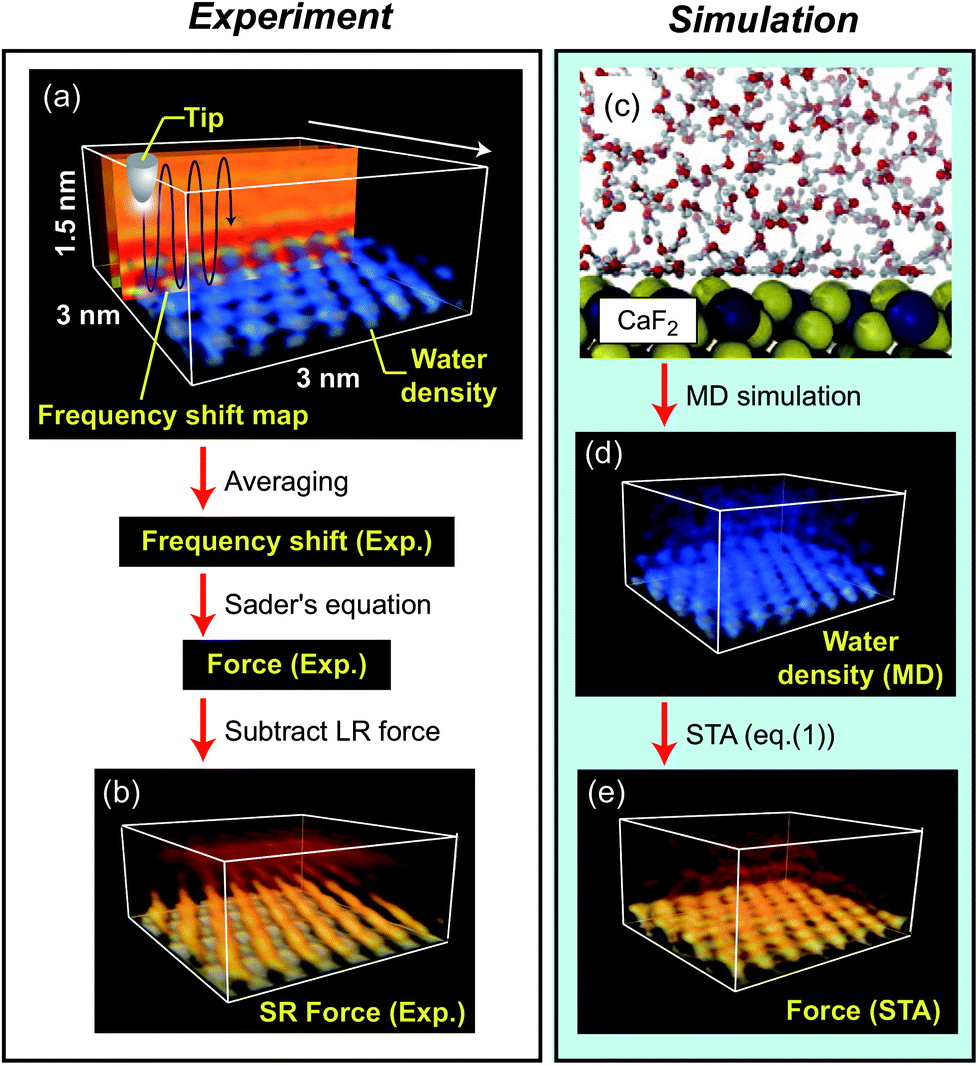
K Miyazawa, N Kobayashi, MW, AL Shluger, K Amano, T Fukuma, Nanoscale 8, 7334 (2016)
Theory: Solvent tip model¶
We don't know the actual tip apex - historical problem with AFM.
Hypothesize that there are likely strongly bound water molecules at the tip apex - and take them as the tip
They feed force back onto the cantilever.
M Watkins, B Reischl, The Journal of chemical physics 138, 154703 (2013)
Statistical mechanical model¶
pure water model leads to
$$ \Delta\Delta G(\mathbf{r}) = -k_BT \ln \frac{\rho(\mathbf{r})}{\rho_{\rm{bulk}}} $$for the free energy change of bringing the tip model (water molecule) from the bulk liquid to $\mathbf{r}$
$$ F(\mathbf{r}) = \frac{\partial \Delta\Delta G(\mathbf{r})}{\partial z} = \frac{k_bT}{\rho(\mathbf{r})}\frac{ \partial \rho(\mathbf{r})}{\partial z} $$this is the 'short range' force exerted on the tip apex.
M Watkins, B Reischl, The Journal of chemical physics 138, 154703 (2013)
Macro vs Nano¶
 |  |
cantilever is macroscopic, tip apex is nanoscopic
experiment measures frequency change due to all interactions - macro + nano
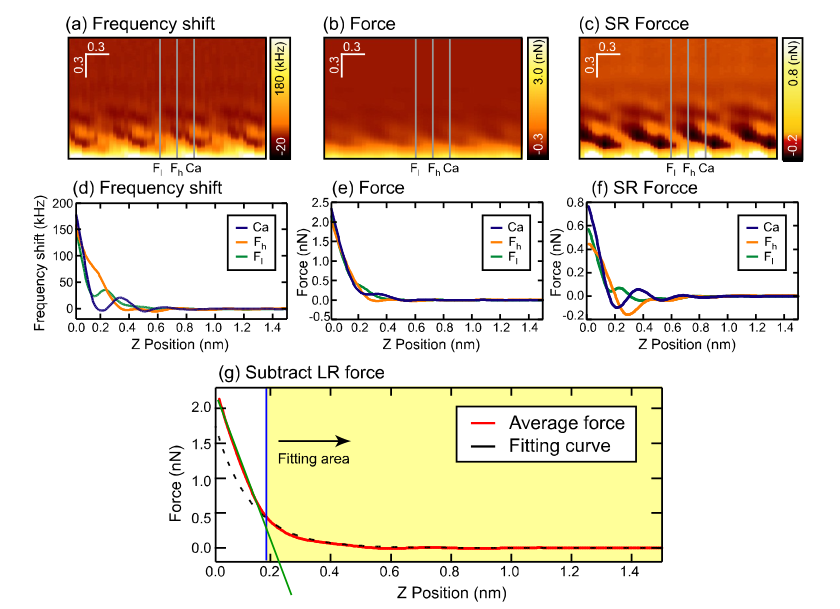
Experiment: Macroscopic effects¶
removed empirically by subtracting force from averaged long range scan data over several surface locations
 |
K Miyazawa, N Kobayashi, MW, AL Shluger, K Amano, T Fukuma, Nanoscale 8, 7334 (2016)
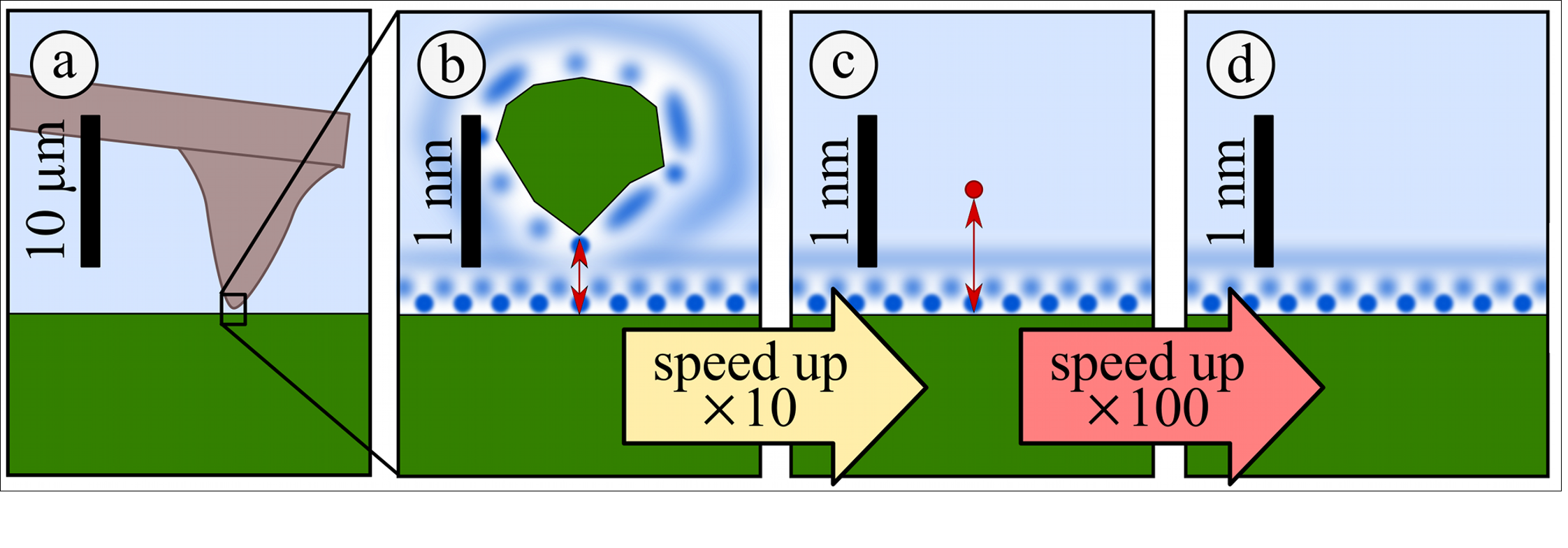
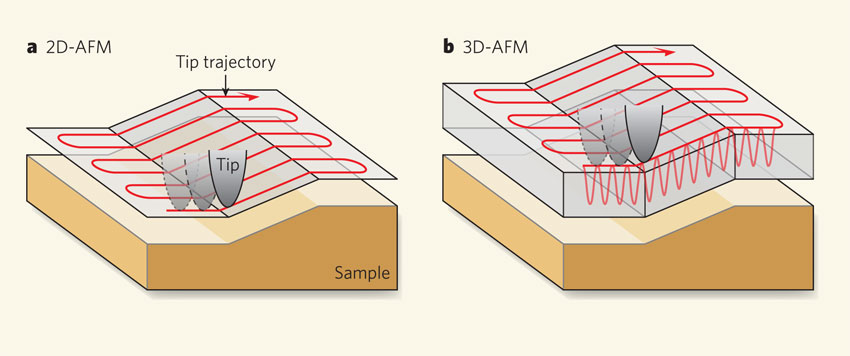
Experiment: fast AFM¶
$\textbf{3D AFM technique}$ and $\textbf{pattern matching}$ routines for massive speed up in image collection efficiency.
 |
 |
Allows image collection within few minutes of exposure of surface to liquid
- (We also use it on the simulation data)
Enables data collection in pure water.
No longer true atomic resolution
K Miyazawa, N Kobayashi, MW, AL Shluger, K Amano, T Fukuma, Nanoscale 8, 7334 (2016)
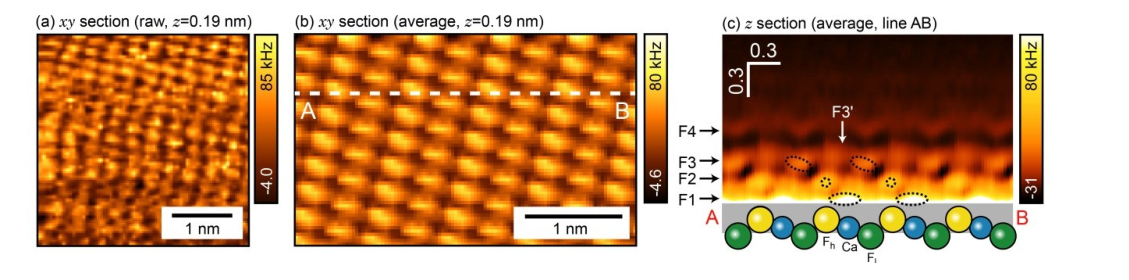
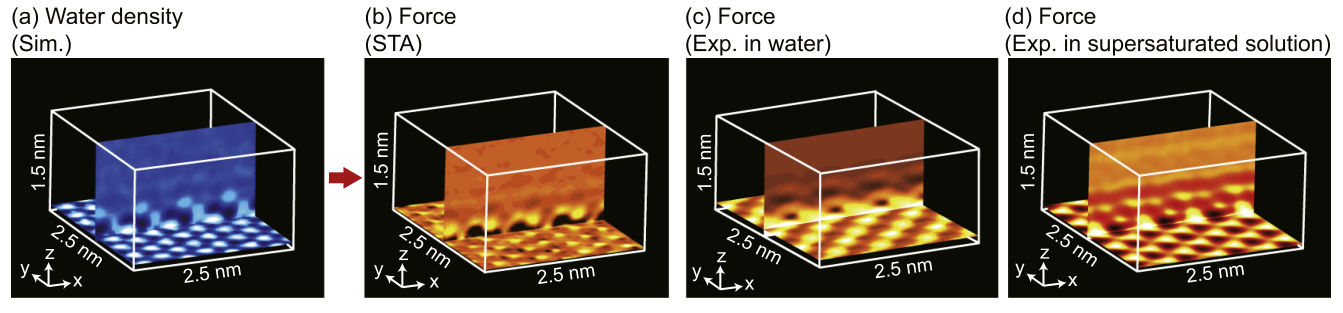
Qualitative comparison of data¶
 | 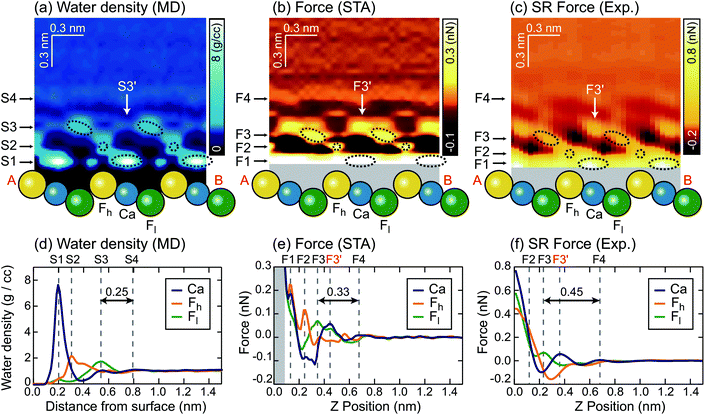 |
K Miyazawa, N Kobayashi, MW, AL Shluger, K Amano, T Fukuma, Nanoscale 8, 7334 (2016)
Can also compare to MD free energy calculation with explicit tips¶
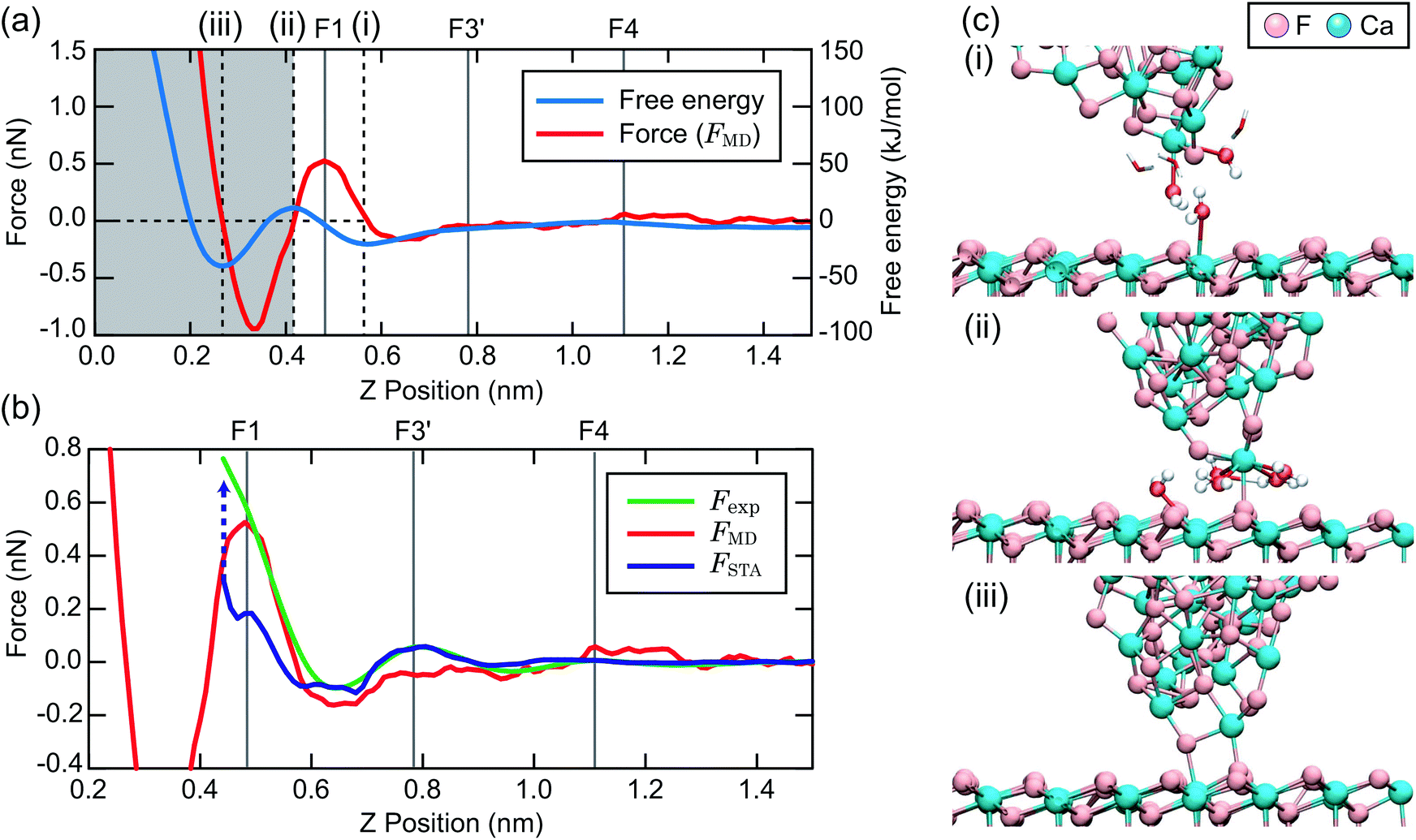
STA does better than an incorrect tip model
K Miyazawa, N Kobayashi, MW, AL Shluger, K Amano, T Fukuma, Nanoscale 8, 7334 (2016)
Realistic environment¶
The above were all obtained in pure water.
requires complicated experimental protocols
not a realistic environment for many of the motivations
Comparison to supersaturated solution¶
- The supersaturated solution ( s = 100 ) was prepared by mixing the same amounts of 38 mM CaCl$_2$ and 76 mM KF solutions
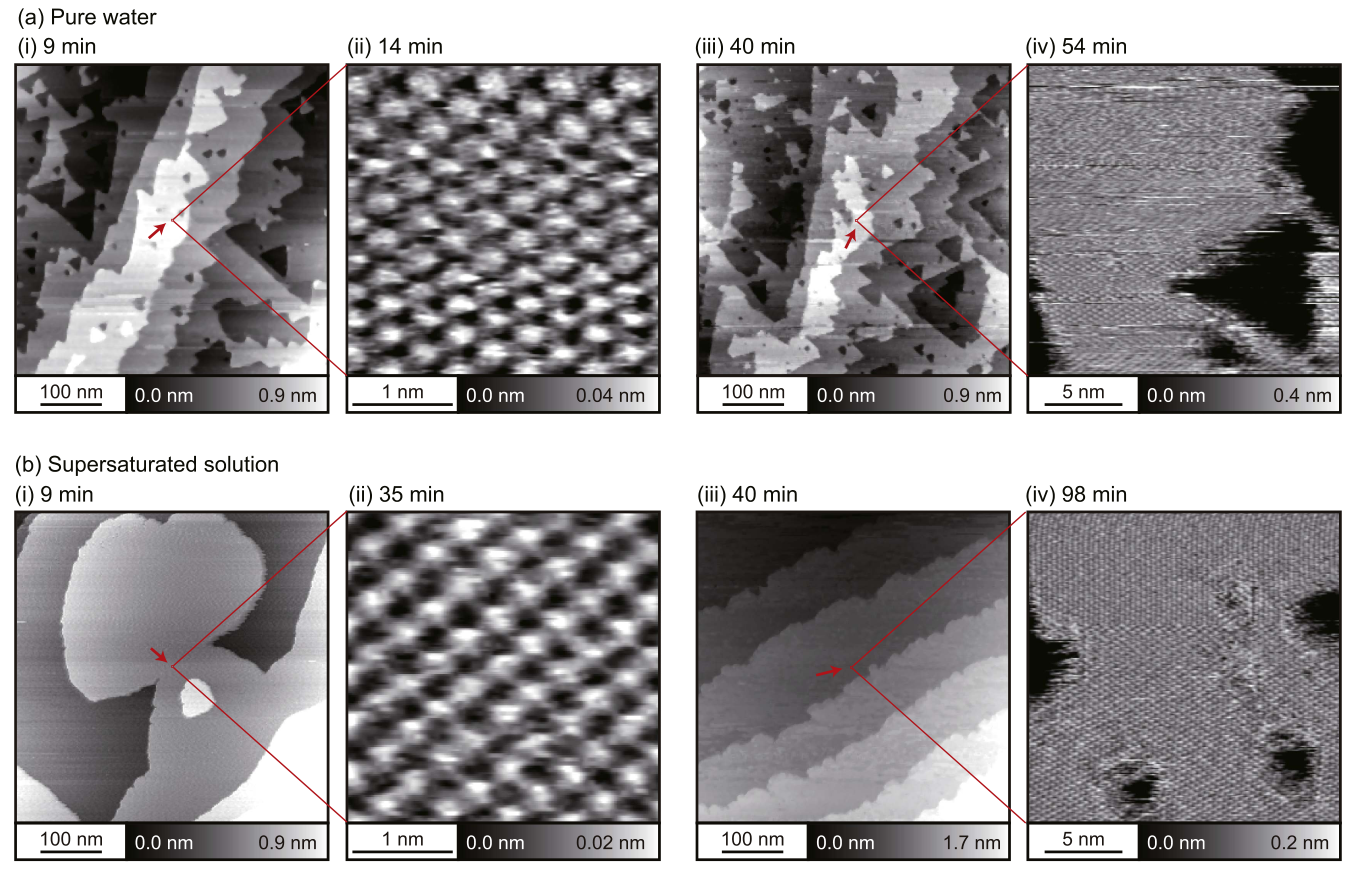
K Miyazawa, MW, AL Shluger, T Fukuma, Nanotechnology 28, 245701 (2017)
Adsorbed ions?¶
- we use the same protocol as before
K Miyazawa, MW, AL Shluger, T Fukuma, Nanotechnology 28, 245701 (2017)
Adsorbed ions?¶
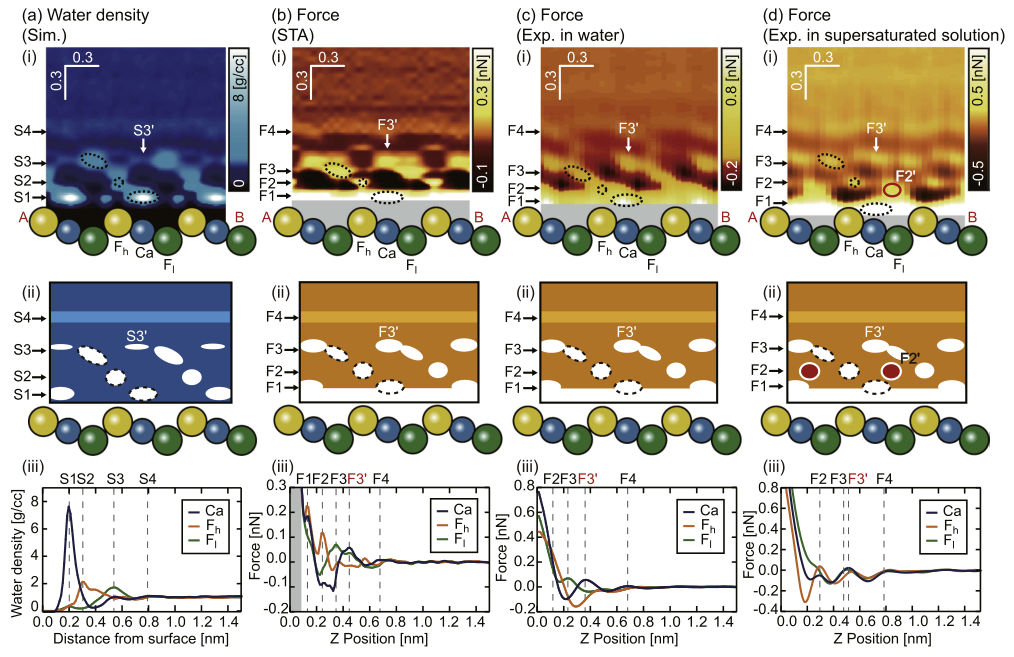
Possible specific cation adorption.
K Miyazawa, MW, AL Shluger, T Fukuma, Nanotechnology 28, 245701 (2017)
Challenge for simulation¶
here is a ~20 ns simulation of the supersaturated solution
- of the order of 1 CaF$_2$ ion complex per 50 nm$^2$
%%HTML
<video width="520" height="500" controls>
<source src="images/F2.mp4" type="video/mp4">
</video>
Conclusions¶
- Evidence suggests here that stable tips are not very invasive – measurement of equilibrium water density
- Theory vs experiment: short range forces above Ca.
- Better agreement between implicit tip model and experiment than explicit MD
- better tip models are needed!
- Towards mapping specific ion adsorption sites and exploring electrolyte solutions
- need to be cleverer than brute force to simulate